Search

ICH -E21 (USFDA) Guidance: Including Pregnant and Breastfeeding Women in Clinical Trials
In May 2025, the FDA, as part of the International Council for Harmonisation (ICH), endorsed and released for consultation the draft E21...

Sharan Murugan
Jul 27, 20253 min read


EMA Guidance: Good Pharmacovigilance Practices (GVP) — Introductory cover note with new Addendum II and GVP Module VI Addendum II – Masking of Personal Data
The European Medicines Agency (EMA) continuously updates its Good Pharmacovigilance Practices (GVP) guidelines to enhance patient privacy...

Sharan Murugan
Jul 27, 20253 min read


Malaysia’s NPRA Guidance: Drug Registration Guidance, What’s Inside?
On 22nd July 2025, Malaysia’s National Pharmaceutical Regulatory Agency (NPRA) released the Third Edition, Tenth Revision of the " Drug...

Sharan Murugan
Jul 23, 20252 min read


USFDA's: FDA’s Commissioner’s National Priority Voucher (CNPV) Pilot Program to Accelerate Life-Changing Medicines
The U.S. Food and Drug Administration (FDA) has launched the " Commissioner’s National Priority Voucher (CNPV) Pilot Program " a...

Sharan Murugan
Jul 23, 20252 min read

EMA Guidance: Requirements for Demonstrating Therapeutic equivalence between Orally Inhaled Products (OIP) and Chronic Obstructive Pulmonary Disease (COPD)
On 14 July 2025 , the European Medicines Agency (EMA) published the final revised guideline titled " Guideline on the requirements for...

Sharan Murugan
Jul 23, 20252 min read


India CDSCO Guidance: Subject Expert Committee (SEC), What Pharma and Clinical Trial Teams Need to Know
The Central Drugs Standard Control Organization (CDSCO) under India’s Ministry of Health & Family Welfare has released a detailed...

Sharan Murugan
Jul 23, 20252 min read


India CDSCO Guidance: How to Get Export NOC for Approved & Unapproved New Drugs
The Central Drugs Standard Control Organisation (CDSCO) has published a fresh guidance document titled “ Guidance Document for Issuance...

Sharan Murugan
Jul 23, 20252 min read


EMA Guidance: Procedural Advice for Orphan Medicinal Product Designation
The European Medicines Agency (EMA) provides structured guidance for sponsors aiming to secure orphan medicinal product designation...

Sharan Murugan
Jul 19, 20252 min read


UK MHRA Guidance: Use of Real-World Data in Clinical Studies to Support Regulatory Decisions (July 2025 Update)
In July 2025, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) released updated and expanded guidance on the use of...

Sharan Murugan
Jul 19, 20253 min read


EMA IRIS Guide: How to Create, Submit and Manage IRIS applications, for Industry and Individual applicants
In the ever-evolving regulatory landscape of EU pharmaceuticals, digital solutions are a linchpin for timely and transparent scientific...

Sharan Murugan
Jul 15, 20252 min read


MHRA’s Guidance: Access, New Active Substance and Biosimilar Work-Sharing Initiatives
In an era of rapid pharmaceutical innovation, global collaboration is key to expediting patient access to new therapies. To help patients...

Sharan Murugan
Jul 15, 20252 min read


Swissmedic Guidnce: A Closer Look at Marketing Authorisation Transfers, DHPC, and RMP Submissions
As the Swiss life sciences sector grows increasingly global and collaborative, Switzerland’s regulatory authority, Swissmedic , continues...

Sharan Murugan
Jul 9, 20252 min read

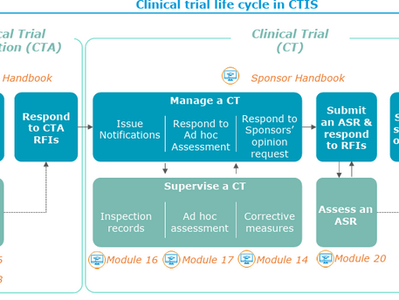
EMA’s CTIS Framework: Decoding the EMA Clinical Trial Information System-A Practical Guide for Sponsors
In an era of data-driven drug development, the European Medicines Agency (EMA) has launched a transformative regulatory framework : the...

Sharan Murugan
Jul 9, 20252 min read


USFDA’s Draft Guidance: Aluminum Content and Labeling for Small Volume Parenteral Drug Products in Parenteral Nutrition
Aluminum contamination in parenteral nutrition (PN) has long posed a silent but serious risk—especially to vulnerable populations like...

Sharan Murugan
Jul 2, 20253 min read


EMA NDSG Work Plan: Inside the Network Data Steering Group Workplan 2025–2028
In an era where data drives decisions and innovation defines progress, the European Medicines Agency (EMA) and Heads of Medicines...

Sharan Murugan
Jul 2, 20253 min read


USFDA Q&A Guidance: Remote Regulatory Assessments (RRA) and Guidance on Antibacterial Therapies (Q&A) and Early Lyme Disease
The U.S. Food and Drug Administration (FDA) has finalized its guidance document on " Conducting Remote Regulatory Assessments (RRAs) ,"...

Sharan Murugan
Jun 29, 20252 min read


USFDA Guidance: UDI Requirements for Combination Products and Cybersecurity for Medical Devices
In June 2025, the FDA released two impactful draft guidances that significantly affect medical device and combination product...

Sharan Murugan
Jun 29, 20253 min read

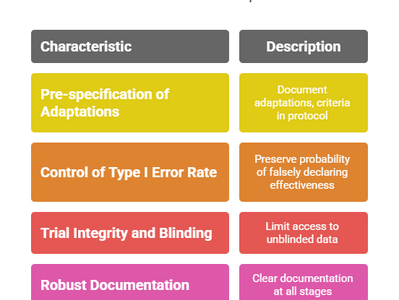
ICH E20 Draft Guideline: Understanding Adaptive Clinical Trials in Focus (2025)
The International Council for Harmonisation (ICH) has taken a major step toward modernizing clinical trial designs with the release of the...

Sharan Murugan
Jun 28, 20253 min read


UK MHRA Guidances: 9 important Guidances on Clinical Trials Safety, Approvals, Labelling, and More
Clinical trials are the cornerstone of modern medicine, ensuring that new treatments are safe, effective, and suitable for public use....

Sharan Murugan
Jun 28, 20253 min read


ICH & USFDA Draft Guidance: Q1 Stability Testing: The Gold Standard for Drug Shelf Life and Quality
The stability of drug substances and drug products is a cornerstone of pharmaceutical quality, ensuring that medicines remain safe,...

Sharan Murugan
Jun 23, 20253 min read
