Search


South Africs's SAHPRA Guidance: on Medical Device Adverse Event Reporting
The South African Health Products Regulatory Authority (SAHPRA) has issued comprehensive guidelines to streamline the reporting of...
Sharan Murugan
Jan 263 min read
13 views
0 comments


UK MHRA Guidance: Strengthening Post-Market Surveillance for Medical Devices
On 15 January, the Medicines and Healthcare products Regulatory Agency (MHRA) released a suite of guidance outlining the new PMS...
Sharan Murugan
Jan 212 min read
26 views
0 comments


UK MHRA Med Dev Guidance: Applying Human Factors to Medical Devices
The Medicines and Healthcare Products Regulatory Agency (MHRA) published its comprehensive guidance titled “ Applying Human Factors and...
Sharan Murugan
Jan 162 min read
9 views
0 comments

USFDA Med Dev Guidance: Premarket Approval Application and Humanitarian Device Exemption Modular Review
The U.S. Food and Drug Administration (FDA) released final guidance " Premarket Approval Application and Humanitarian Device Exemption...
Sharan Murugan
Jan 162 min read
10 views
0 comments


USFDA Guidance: Developing Drugs for Optical Imaging – A Comprehensive Insight
On 07 January 2025, the U.S. Food and Drug Administration (FDA) released the draft guidance titled " Developing Drugs for Optical Imaging...
Sharan Murugan
Jan 112 min read
17 views
0 comments


USFDA Guidance Updates: A Comprehensive Look at Recent Policies and Draft Recommendations
The U.S. Food and Drug Administration (FDA) has recently issued several key guidance documents addressing critical areas in...
Sharan Murugan
Jan 73 min read
31 views
0 comments


USFDA Guidance: Artificial Intelligence-Enabled Device Software Functions – Lifecycle Management and Marketing Submission Recommendations
The U.S. Food and Drug Administration (FDA) has issued a draft guidance document titled “ Artificial Intelligence-Enabled Device Software...
Sharan Murugan
Jan 72 min read
19 views
0 comments


USFDA Guidance: Protocol Deviations for Clinical Investigations of Drugs, Biological Products, and Devices
The U.S. Food and Drug Administration (FDA) recently released (26 December 2024) draft guidance titled “ Protocol Deviations for Clinical...
Sharan Murugan
Dec 28, 20242 min read
105 views
0 comments


USFDA Guidance: Global Unique Device Identification Database (GUDID): Enhancing Medical Device Transparency
The U.S. Food and Drug Administration (FDA) has updated and released its " Global Unique Device Identification Database (GUDID) " final...
Sharan Murugan
Dec 21, 20242 min read
30 views
0 comments


SAHPRA's: Guidelines for Medical Device Adverse Event Reporting
The South African Health Products Regulatory Authority (SAHPRA) has released two crucial guidelines to streamline adverse event reporting...
Sharan Murugan
Dec 15, 20242 min read
25 views
0 comments


TGA: Australian Regulatory Guidelines for Medical Devices (ARGMD)
The Australian Regulatory Guidelines for Medical Devices (ARGMD) , issued by the Therapeutic Goods Administration (TGA), provide a...
Sharan Murugan
Dec 15, 20242 min read
79 views
0 comments

USFDA Final Guidance: Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions
The US Food and Drug Administration (USFDA) has issued its final guidance on " Marketing Submission Recommendations for a Predetermined...
Sharan Murugan
Dec 9, 20242 min read
16 views
0 comments

USFDA Guidance: Enhancing Communication and Guidance Development Practices
The US Food and Drug Administration (USFDA) has released two insightful reports aimed at improving its internal practices and...
Sharan Murugan
Dec 4, 20242 min read
22 views
0 comments


UK Med Dev Guidance: Clinical Investigations for Medical Devices
The Medicines and Healthcare products Regulatory Agency (MHRA) has published detailed guidance " Clinical Investigation s " on...
Sharan Murugan
Dec 4, 20242 min read
58 views
0 comments


UK MHRA Press Release Trials Innovative AI Technologies in Regulatory Pilot Scheme
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has launched (4th December 2024) a groundbreaking pilot scheme "...
Sharan Murugan
Dec 4, 20242 min read
28 views
0 comments

MDCG Guidance: Implementation of the Master UDI-DI Solution for Contact Lenses
In November 2024, the Medical Device Coordination Group (MDCG) released " MDCG 2024-14 - Guidance on the implementation of the Master...
Sharan Murugan
Nov 30, 20242 min read
12 views
0 comments


USFDA MD Guidance: Enhancing Safety and Efficiency in Orthopedic Devices and Sterilization Processes
The USFDA Center for Devices and Radiological Health has recently issued three key final guidance documents focusing on orthopedic...
Sharan Murugan
Nov 27, 20242 min read
11 views
0 comments

Malaysia’s Medical Device Authority (MDA) Guidance: Control of Obsolete and Discontinued Medical Devices in Healthcare or Related Facilities
The Medical Device Authority (MDA) in Malaysia has released a comprehensive guidance " Control of Obsolete and Discontinued Medical...
Sharan Murugan
Nov 20, 20242 min read
17 views
0 comments
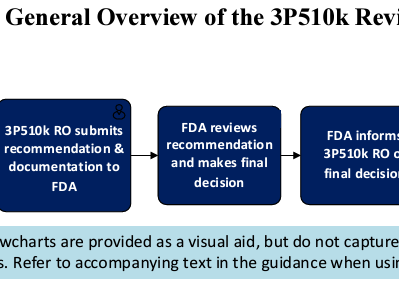
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...
Sharan Murugan
Nov 20, 20242 min read
22 views
0 comments


Swissmedic Guidance: Combined Studies: Clinical Investigations of Medical Devices, Medicinal Products, and Advanced Therapy Medicinal Products (ATMPs)
The Swissmedic guidance released an information sheet titled " Combined Studies: Clinical Investigations of Medical Devices, Medicinal...
Sharan Murugan
Nov 9, 20242 min read
15 views
0 comments
