Search


ICH Seeks Market Consultation for Global Post-Approval CMC Assessment Cloud Solution
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has issued a Request for...

Sharan Murugan
Nov 30, 20242 min read


MDCG Guidance: Implementation of the Master UDI-DI Solution for Contact Lenses
In November 2024, the Medical Device Coordination Group (MDCG) released " MDCG 2024-14 - Guidance on the implementation of the Master...

Sharan Murugan
Nov 30, 20242 min read


EMA Guidance: Scientific Guidelines with Summary of Product Characteristics (SmPC) Recommendations
The European Medicines Agency (EMA) has released a comprehensive document " Scientific guidelines with summary-of-product-characteristi...

Sharan Murugan
Nov 30, 20242 min read


UK MHRA Issues Final Call to Comply with Windsor Framework for Medicines by January 2025
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has issued " Call to comply with Windsor Framework arrangements for...

Sharan Murugan
Nov 29, 20242 min read


USFDA: Guidances to Advance Drug Development and Safety Assessments Advance Drug Development and Safety Assessments
The USFDA continues to innovate its regulatory landscape with the release of three important guidance earlier today (27 November, 2024)...

Sharan Murugan
Nov 27, 20242 min read


USFDA MD Guidance: Enhancing Safety and Efficiency in Orthopedic Devices and Sterilization Processes
The USFDA Center for Devices and Radiological Health has recently issued three key final guidance documents focusing on orthopedic...

Sharan Murugan
Nov 27, 20242 min read


Malaysia’s Medical Device Authority (MDA) Guidance: Control of Obsolete and Discontinued Medical Devices in Healthcare or Related Facilities
The Medical Device Authority (MDA) in Malaysia has released a comprehensive guidance " Control of Obsolete and Discontinued Medical...

Sharan Murugan
Nov 20, 20242 min read


Australia's TGA: Listed Medicines Evidence Guidelines
Earlier today (20 November, 2024) the Therapeutic Goods Administration (TGA) released Version 4.0 of its Listed Medicines " Evidence...

Sharan Murugan
Nov 20, 20242 min read

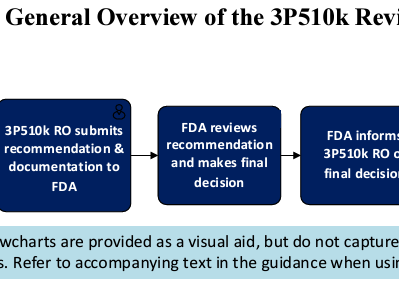
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...

Sharan Murugan
Nov 20, 20242 min read


Swissmedic Guidance on Product Information for Human Medicinal Products
Swissmedic, the Swiss Agency for Therapeutic Products, has introduced its updated guidance on " Product Information for Human Medicinal...

Sharan Murugan
Nov 16, 20242 min read


USFDA Guidance: Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutics
This USFDA draft guidance" Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutic s" , issued on 15 November 2024, offers...

Sharan Murugan
Nov 16, 20242 min read


UK MHRA Guidance: How Marketing Authorisation Applications referred under Article 29 are Handled
The UK MHRA updated its Guidance on " How Marketing Authorisation Applications referred under Article 29 are Handled " and outlines the...

Sharan Murugan
Nov 16, 20242 min read


South Africa's SAHPRA: Communication to Industry on Quality Variations
The SAHPRA released a " Communication to Industry on Quality Variations " document that addresses updates on the submission requirements...

Sharan Murugan
Nov 11, 20242 min read


South Africa-SAHPRA: Engagement Portal
As part of the South African Health Product Regulatory Authority’s (SAHPRA) Digitalisation Project, we launched the SAHPRA Engagement...

Sharan Murugan
Nov 9, 20242 min read


Swissmedic Guidance: Combined Studies: Clinical Investigations of Medical Devices, Medicinal Products, and Advanced Therapy Medicinal Products (ATMPs)
The Swissmedic guidance released an information sheet titled " Combined Studies: Clinical Investigations of Medical Devices, Medicinal...

Sharan Murugan
Nov 9, 20242 min read
