Search


UK MHRA: Guidance on Register Medical Devices to Place on the Market
Last Friday on 1st December 2023 UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Register...
Sharan Murugan
Dec 3, 20231 min read
20 views
0 comments


Health Canada: Guidance on Validation rules for Regulatory Transactions in the eCTD Format
Health Canada recently published an updated guidance on "Validation rules for regulatory transactions provided to Health Canada in the...
Sharan Murugan
Nov 30, 20231 min read
73 views
0 comments


Australia TGA: Clinical Evidence Guidelines
The Australian Regulatory Guidelines for Medical Devices (ARGMD) released updated guidance "Clinical Evidence" on 23 November 2023,...
Sharan Murugan
Nov 26, 20232 min read
36 views
0 comments


Swiss Medic: Biosimilar Authorisation Guidance
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance on (26 November 2023) the "Guidance document...
Sharan Murugan
Nov 26, 20232 min read
42 views
0 comments


South Africa's (SAPHRA): Questions And Answers Licensing Of Medical Device Establishments
Last Friday (17 November 2023) the South African Health Products Regulatory Authority (SAPHRA) released updated guidance on "Guideline On...
Sharan Murugan
Nov 19, 20231 min read
36 views
0 comments


USFDA MD Guidance: Notifying FDA of a Permanent Discontinuance, 506J Guidance & Computational Model
USFDA's Center for Devices and Radiological Health and Center for Biologics Evaluation and Research released multiple guidances related...
Sharan Murugan
Nov 19, 20232 min read
15 views
0 comments


UK MHRA: Guidance on Change of Ownership: Marketing Authorisation Process
Last Friday (17 November 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Medicines...
Sharan Murugan
Nov 19, 20232 min read
191 views
0 comments

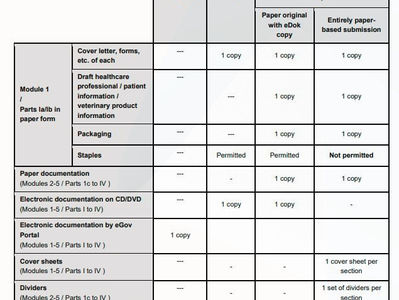
Switzerland's SwissMedic: Guidance on Formal Requirements
On 15 November 2023, Switzerland's Swissmedic released an updated guidance on "Formal Requirements" which serves as a resource for...
Sharan Murugan
Nov 15, 20232 min read
44 views
0 comments


USFDA Guidance: Real-Time Oncology Review (RTOR)
The U.S. Food and Drug Administration (FDA) Oncology Center of Excellence (OCE) released guidance earlier this week (07 November 2023)...
Sharan Murugan
Nov 11, 20232 min read
25 views
0 comments


MHRA Guidance: Common Issues Identified during Clinical Trial Applications
Yesterday (06 November 2023) the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Common...
Sharan Murugan
Nov 7, 20231 min read
43 views
0 comments


USFDA Guidance: Submitting Patient-Reported Outcome Data, Clinical Trial Datasets & Documentation
Today (06 November, 2023) the U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) released two final...
Sharan Murugan
Nov 6, 20232 min read
16 views
0 comments


USFDA MD Guidance: Enforcement Policy for Clinical Electronic Thermometers
Last Friday (03 November 2023) the U.S. Food and Drug Administration (FDA) released two draft guidance "Enforcement Policy for Clinical...
Sharan Murugan
Nov 5, 20231 min read
9 views
0 comments


USFDA Guidance: Supplements for Approved Premarket Approval or Humanitarian Device Exemption
Today (02 November, 2023) the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research Center for Devices and...
Sharan Murugan
Nov 2, 20231 min read
20 views
0 comments


TGA Guidance: General Dossier Requirements
Recently on 27th October, 2023 Australia's Therapeutic Goods Administration (TGA) released an updated guidance on the "General Dossier...
Sharan Murugan
Oct 31, 20231 min read
226 views
0 comments


UK MHRA: Guidance on Software and Artificial Intelligence (AI) as a Medical Device
Earlier today (25 October 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated Guidance on "Software...
Sharan Murugan
Oct 25, 20231 min read
43 views
0 comments


USFDA: Remote Interactive Evaluations of Drug Manufacturing & Bioresearch Monitoring Facilities
Earlier today (25 October,2023), the USFDA issued a draft guidance, "Remote Interactive Evaluations of Drug Manufacturing and Bioresearch...
Sharan Murugan
Oct 25, 20231 min read
23 views
0 comments


USFDA MD Guidance: Enforcement Policy for Non-Invasive Remote Monitoring Devices
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released a final guidance "Enforcement Policy for...
Sharan Murugan
Oct 23, 20231 min read
18 views
0 comments


USFDA Guidance: Benefit-Risk Assessment for New Drug and Biological Products
A final guidance "Benefit-Risk Assessment for New Drug and Biological Products" was published last week (17 October, 2023) by the US Food...
Sharan Murugan
Oct 23, 20232 min read
29 views
0 comments


EMA Guidance: Procedural Advice for Orphan Medicinal Product Designation
Yesterday (20 October 2023) the European Medicines Agency released an updated guidance on "Procedural Advice for Orphan Medicinal Product...
Sharan Murugan
Oct 21, 20232 min read
33 views
0 comments


UK MHRA Guidance: Apply for a Licence to Market a Medicine in the UK
Last Thursday (19 October 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Apply for a...
Sharan Murugan
Oct 21, 20231 min read
61 views
0 comments
