Search


SFDA Guidance: Requirements for Formal Meetings Between the Drug Sector and Applicants
Th e Saudi Food and Drug Authority (SFDA) has issued an updated version (3.1, dated 7 April 2025) of its guideline: " Requirements for...

Sharan Murugan
9 hours ago2 min read
1 view
0 comments


MDCG Guidance: MDR requirements for Legacy Devices
The Medical Device Coordination Group (MDCG) has released guidance " Application of MDR requirements to ‘legacy devices’ and to devices...

Sharan Murugan
Oct 27, 20242 min read
41 views
0 comments


India CDSCO: Guidance for Industry on Pharmacovigilance Requirements for Human Vaccines
The Central Drugs Standard Control Organisation (CDSCO) has issued comprehensive guidance on (29 May, 2024) "Guidance for Industry on...

Sharan Murugan
Jun 5, 20242 min read
17 views
0 comments


Australia TGA: Regulatory requirements for in-house IVDs
Today (01 May 2024) Australia's Therapeutic Goods Administration (TGA) released updated guidance "Regulatory requirements for in-house...

Sharan Murugan
May 1, 20242 min read
23 views
0 comments


Saudi Arabia’s (SFDA): Requirements for Clinical Trials of Medical Devices
The Saudi Food and Drug Authority (SFDA) issued a comprehensive updated guidance titled "Requirements for Clinical Trials of Medical...

Sharan Murugan
Dec 30, 20231 min read
35 views
0 comments

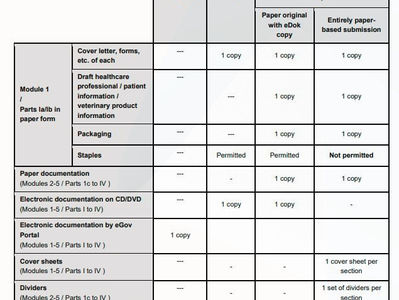
Switzerland's SwissMedic: Guidance on Formal Requirements
On 15 November 2023, Switzerland's Swissmedic released an updated guidance on "Formal Requirements" which serves as a resource for...

Sharan Murugan
Nov 15, 20232 min read
45 views
0 comments


Health Canada: Guidance on Master Files Procedures & Administrative Requirements
Health Canada recently (26 June, 2023) published an updated guidance on "Guidance on Procedures and Administrative Requirements for...

Sharan Murugan
Jul 2, 20231 min read
164 views
0 comments


Saudi Arabia’s (SFDA): Requirements on Importation and Shipments of Medical Devices (MDS-REQ5)
Earlier today (21 May 2023) to ensure the importation and shipment of medical devices comply with rigorous standards, the Saudi Food and...

Sharan Murugan
May 21, 20231 min read
177 views
0 comments


SFDA MD Guidance: Requirements for Clinical Trials of Medical Device
Yesterday (26-December-2022) the South African Health Products Regulatory Authority (SAPHRA) released an updated "Requirements for...

Sharan Murugan
Dec 27, 20222 min read
134 views
0 comments
