Search


Swissmedic Guidance: Import of a Human Medicinal Product (Parallel Import)
Today (01 May 2024) Switzerland's Swissmedic released updated guidance on "Import of a Human Medicinal Product according to Art. 14 para....

Sharan Murugan
May 1, 20242 min read
18 views
0 comments


Switzerland's SwissMedic: Guidance on Export Certificates
On 3rd April, 2024 Switzerland's Swissmedic released an updated "Guidance on Export Certificates" and as part of its regulatory...

Sharan Murugan
Apr 7, 20242 min read
70 views
0 comments


Swiss Medic: eCTD Guidance for Industry & Time limits for Authorisation Applications
Swissmedic, recently published updated guidance on "Guidance Industry eCTD" and "Guidance on Time limits for Authorisation Applications"....

Sharan Murugan
Mar 29, 20242 min read
69 views
0 comments


Swissmedic Guidance: Navigating Variations, Product Information, and Electronic ICSRs through PV Gateway
Yesterday (06 February 2024) Switzerland's Swissmedic released updated guidance on "Electronic exchange of ICSRs through PV Gateway", and...

Sharan Murugan
Feb 7, 20242 min read
93 views
0 comments


Swiss Medic: Biosimilar Authorisation Guidance
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance on (26 November 2023) the "Guidance document...

Sharan Murugan
Nov 26, 20232 min read
42 views
0 comments

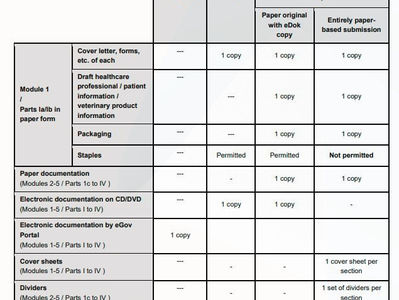
Switzerland's SwissMedic: Guidance on Formal Requirements
On 15 November 2023, Switzerland's Swissmedic released an updated guidance on "Formal Requirements" which serves as a resource for...

Sharan Murugan
Nov 15, 20232 min read
45 views
0 comments


Switzerland's SwissMedic: Harmonisation of the SwissGMP/GDP Inspection System
Yesterday (20 July 2023) Switzerland's Swissmedic released an updated guidance on "Harmonisation of the SwissGMP/GDP Inspection System"...

Sharan Murugan
Jul 21, 20232 min read
48 views
0 comments


Switzerland's SwissMedic: Guidance on Medicinal Product Name
Yesterday (07 July 2023) Switzerland's Swissmedic released an updated guidance on "Medicinal Product Name" that describes the...

Sharan Murugan
Jul 8, 20231 min read
35 views
0 comments


Switzerland's SwissMedic: Guidance on Authorisation Biosimilar
Yesterday (21 June 2023) Switzerland's Swissmedic released an updated guidance on "Authorisation Biosimilar" which specifies the...

Sharan Murugan
Jun 22, 20231 min read
36 views
0 comments


SwissMedic Guidance: Orphan Drug, New Active Substance & Prior Notification
Swissmedic recently (02 June 2023) released multiple important guidelines that have captivated the audience and generated significant...

Sharan Murugan
Jun 4, 20232 min read
46 views
0 comments


SwissMedic Guidance on Fast-Track Authorisation, Temporary Authorisation and Variations & Extensions
Last week Swissmedic, the Swiss Agency for Therapeutic Products updated and released 3 Important guidances and forms. 1. Guidance on...

Sharan Murugan
Jun 4, 20231 min read
34 views
0 comments


Swiss Medic Guidance: Minimising the Risk of TSE and Authorisation of Radiopharmaceuticals
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance document on (26 May 2023) the "Guidance document...

Sharan Murugan
May 28, 20232 min read
34 views
0 comments


Switzerland's Swissmedic: Med Dev Guidance on Export Certificates and Service Agreement
Yesterday (23 May 2023) Switzerland's Swissmedic released two updated guidances one is "Export Certificates" guidance and another one is...

Sharan Murugan
May 23, 20232 min read
35 views
0 comments


Swiss Medic: Guidance on Transfer of Marketing Authorisation
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance document on (3 May 2023) the "Transfer of Marketing...

Sharan Murugan
May 7, 20231 min read
52 views
0 comments


Swiss Medic: Information sheet on Clinical Investigations with Medical Devices
Recently (13-April-2023) Switzerland's Swissmedic released an updated Information sheet document for "Information sheet on Clinical...

Sharan Murugan
Apr 17, 20231 min read
14 views
0 comments


Swissmedic: eCTD v4.0 Implementation Guide published
Earlier today (17 March 2023) Switzerland's SwissMedic released published the "Implementation Guide for eCTD v4.0" and the implementation...

Sharan Murugan
Mar 17, 20231 min read
176 views
0 comments


Swissmedic Guidance: Requesting Certificate of a Pharmaceutical Product (CPP)
Earlier today (13 March 2023) Switzerland's SwissMedic released an updated "Guidance document for requesting product certificates (CPP)"...

Sharan Murugan
Mar 13, 20231 min read
98 views
0 comments


Swiss Medic Guidance: Product Information & Packaging for Human Medicinal Products
Recently (01 march, 2023) Swissmedic released an updated guidance document on "Product information for human medicinal products" and...

Sharan Murugan
Mar 4, 20231 min read
49 views
0 comments


Swiss Medic Guidance: Document on Formal requirements
Swissmedic released an updated guidance document on "Formal requirements" yesterday (15 February 2023). By publishing this document,...

Sharan Murugan
Feb 16, 20231 min read
84 views
0 comments


Swissmedic Guidance: Temporary Authorisation to Use an Unauthorised Medicinal Product
A temporary authorisation of use of an unauthorised medicinal product under restricted conditions can be granted to the sponsor of a...

Sharan Murugan
Dec 27, 20221 min read
41 views
0 comments
