Search
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
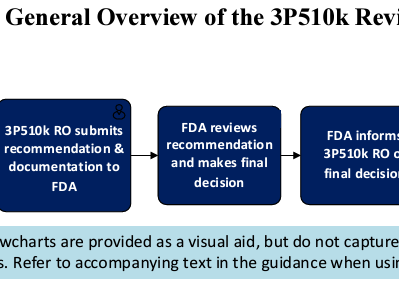
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...
Sharan Murugan
Nov 20, 20242 min read
22 views
0 comments
