Search

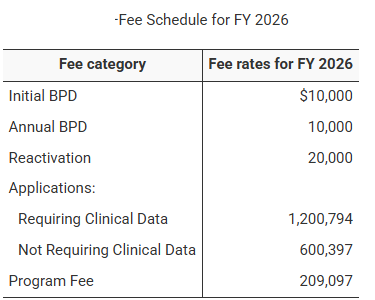
USFDA Announcement: Biosimilar User Fee Rates for Fiscal Year 2026 (October 1, 2025 – September 30, 2026)
On 30 July 2025, the U.S. Food and Drug Administration (FDA) officially published the Biosimilar User Fee Rates for Fiscal Year (FY) 2026...

Sharan Murugan
Aug 2, 20252 min read


USFDA Med Dev Guidance: Medical Device User Fee Small Business Qualification and Determination
In July 2025, the USFDA published a revised guidance titled " Medical Device User Fee Small Business Qualification and Determination "...

Sharan Murugan
Jul 31, 20252 min read


USFDA Guidance: Formal Meetings Between the FDA and Sponsors or Applicants of BsUFA Products
In July 2025, the U.S. Food and Drug Administration (FDA) published its updated guidance for industry titled “ Formal Meetings Between...

Sharan Murugan
Jul 19, 20253 min read


USFDA Guidance: Drug Development for Myelodysplastic Syndromes (MDS)
Myelodysplastic syndromes (MDS) are a group of complex, clonal blood disorders that predominantly affect older adults and may progress to...

Sharan Murugan
Jul 5, 20252 min read


USFDA Guidance: Post-Warning Letter Meetings Under GDUFA III: A Regulatory Pathway Toward Compliance
In the evolving regulatory landscape of generic drug manufacturing, compliance and transparency are more crucial than ever. The FDA’s...

Sharan Murugan
Jun 18, 20252 min read


USFDA Guidance: ANDAs: Pre-Submission Facility Correspondence Related to Prioritized Generic Drug Submissions
The U.S. Food and Drug Administration (FDA) has released an updated guidance " ANDAs: Pre-Submission Facility Correspondence Related to...

Sharan Murugan
Jun 13, 20252 min read


USFDA Draft Med.Dev Q&A: Transfer of a Premarket Notification (510(k)) Clearance – Questions and Answers
In June 2025, the U.S. Food and Drug Administration (FDA) released a new draft guidance titled “ Transfer of a Premarket Notification...

Sharan Murugan
Jun 7, 20252 min read


USFDA Guidance: M11 Technical Specification & Template: Clinical Electronic Structured Harmonised Protocol (CeSHarP)
In a major step toward global harmonisation of clinical trial processes, the U.S. Food and Drug Administration (FDA) and the...

Sharan Murugan
Jun 7, 20252 min read


Meet ELSA: USFDA Launches Agency-Wide AI Tool to Optimize Performance
What if the agonizing wait for drug approvals could be slashed from days to minutes? Imagine a future where breakthrough therapies reach...

Sharan Murugan
Jun 2, 20252 min read


USFDA’s Draft Guidance: Bioequivalence Biowaivers for Additional Strengths of Immediate-Release Oral Drugs
Developing drug products across multiple strengths is a common strategy in pharmaceutical formulation, allowing dose flexibility and...

Sharan Murugan
May 30, 20252 min read


USFDA’s Med Dev Guidance: The Q-Submission Program & Electronic Submission Template for Medical Device Q-Submissions
As medical technology advances, the FDA’s regulatory frameworks continue evolving to ensure timely, safe, and effective device...

Sharan Murugan
May 30, 20252 min read


USFDA Draft Guidance: Replacing Color Additives in Approved or Marketed Drug Products
A color additive is any dye, pigment, or substance that imparts color to a drug. Only color additives listed in FDA regulations are...

Sharan Murugan
May 30, 20252 min read


USFDA Announcement: Shortening the Drug Approval Process- Integration of Generative AI by June end & Completion of First AI-Assisted Scientific Review
The U.S. Food and Drug Administration (FDA) has marked a significant milestone in its digital transformation journey by announcing the...

Sharan Murugan
May 13, 20252 min read


USFDA Guidance: Evaluation of Sex-Specific Data in Medical Device Clinical Studies (March 2025)
In March 2025, the U.S. Food and Drug Administration (FDA) released a final guidance titled “ Evaluation of Sex-Specific Data in Medical...

Sharan Murugan
Apr 5, 20252 min read


USFDA Announcement: Facility Fee Rates for OTC Monograph Drug User Fee Program (OMUFA) for FY 2025
The U.S. Food and Drug Administration (FDA) has announced the release of " Over-the-Counter Monograph Drug User Fee Program-Facility Fee...

Sharan Murugan
Mar 21, 20252 min read


UK MHRA Med Dev Guidance: Applying Human Factors to Medical Devices
The Medicines and Healthcare Products Regulatory Agency (MHRA) published its comprehensive guidance titled “ Applying Human Factors and...

Sharan Murugan
Jan 16, 20252 min read


USFDA Med Dev Guidance: Premarket Approval Application and Humanitarian Device Exemption Modular Review
The U.S. Food and Drug Administration (FDA) released final guidance " Premarket Approval Application and Humanitarian Device Exemption...

Sharan Murugan
Jan 16, 20252 min read


USFDA Guidance: Considerations for Complying with 21 CFR 211.110
The US Food and Drug Administration (FDA) has released the Draft Guidance document titled " Considerations for Complying with 21 CFR...

Sharan Murugan
Jan 6, 20252 min read


USFDA Guidance: Protocol Deviations for Clinical Investigations of Drugs, Biological Products, and Devices
The U.S. Food and Drug Administration (FDA) recently released (26 December 2024) draft guidance titled “ Protocol Deviations for Clinical...

Sharan Murugan
Dec 28, 20242 min read


USFDA Guidance: Global Unique Device Identification Database (GUDID): Enhancing Medical Device Transparency
The U.S. Food and Drug Administration (FDA) has updated and released its " Global Unique Device Identification Database (GUDID) " final...

Sharan Murugan
Dec 21, 20242 min read
