Search


USFDA Guidance: Evaluation of Sex-Specific Data in Medical Device Clinical Studies (March 2025)
In March 2025, the U.S. Food and Drug Administration (FDA) released a final guidance titled “ Evaluation of Sex-Specific Data in Medical...
Sharan Murugan
6 days ago2 min read
9 views
0 comments


USFDA Announcement: Facility Fee Rates for OTC Monograph Drug User Fee Program (OMUFA) for FY 2025
The U.S. Food and Drug Administration (FDA) has announced the release of " Over-the-Counter Monograph Drug User Fee Program-Facility Fee...
Sharan Murugan
Mar 212 min read
70 views
2 comments


USFDA Guidance: Recommendations to Reduce the Risk of Transmission of Disease Agents Associated with Sepsis and Reduce the Risk of Transmission of Mycobacterium tuberculosis
The U.S. Food and Drug Administration (USFDA) has released two important guidance documents addressing the risks of sepsis and...
Sharan Murugan
Feb 13 min read
19 views
0 comments


USFDA Guidance: Bioanalytical Method Validation for Biomarkers
On 21st January 2025, the U.S. Food and Drug Administration's (FDA) Center for Drug Evaluation and Research issued final guidance "...
Sharan Murugan
Jan 212 min read
286 views
0 comments


UK MHRA Med Dev Guidance: Applying Human Factors to Medical Devices
The Medicines and Healthcare Products Regulatory Agency (MHRA) published its comprehensive guidance titled “ Applying Human Factors and...
Sharan Murugan
Jan 162 min read
8 views
0 comments

USFDA Med Dev Guidance: Premarket Approval Application and Humanitarian Device Exemption Modular Review
The U.S. Food and Drug Administration (FDA) released final guidance " Premarket Approval Application and Humanitarian Device Exemption...
Sharan Murugan
Jan 162 min read
10 views
0 comments


USFDA Guidance: Considerations for Complying with 21 CFR 211.110
The US Food and Drug Administration (FDA) has released the Draft Guidance document titled " Considerations for Complying with 21 CFR...
Sharan Murugan
Jan 62 min read
22 views
0 comments


Latest ICH Guidance updates: M15-Model-Informed Drug Development, E6(R3)-Good Clinical Practice: Annex 2, & E11A -Pediatric Extrapolation
The International Council for Harmonisation (ICH) continues to advance global standards in drug development and clinical practices with...
Sharan Murugan
Dec 28, 20242 min read
28 views
0 comments


USFDA Guidance: Protocol Deviations for Clinical Investigations of Drugs, Biological Products, and Devices
The U.S. Food and Drug Administration (FDA) recently released (26 December 2024) draft guidance titled “ Protocol Deviations for Clinical...
Sharan Murugan
Dec 28, 20242 min read
102 views
0 comments


USFDA Guidance: Global Unique Device Identification Database (GUDID): Enhancing Medical Device Transparency
The U.S. Food and Drug Administration (FDA) has updated and released its " Global Unique Device Identification Database (GUDID) " final...
Sharan Murugan
Dec 21, 20242 min read
30 views
0 comments


USFDA: CDER Establishes New Center for Real-World Evidence Innovation (CCRI)
The U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) has unveiled an innovative initiative—the CDER...
Sharan Murugan
Dec 15, 20242 min read
10 views
0 comments


USFDA Guidance: Standardized Format for Electronic Submission of NDA and BLA Content for Bioresearch Monitoring (BIMO) Inspections
The United States Food and Drug Administration (USFDA) has released detailed guidance" Standardized Format for Electronic Submission of...
Sharan Murugan
Dec 9, 20242 min read
33 views
0 comments


USFDA Draft Guidance: Accelerated Approval – Expedited Program for Serious Conditions
The United States Food and Drug Administration (USFDA) has released (05 December, 2024) the draft guidance " Expedited Program for...
Sharan Murugan
Dec 9, 20242 min read
18 views
0 comments

USFDA Final Guidance: Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions
The US Food and Drug Administration (USFDA) has issued its final guidance on " Marketing Submission Recommendations for a Predetermined...
Sharan Murugan
Dec 9, 20242 min read
16 views
0 comments

USFDA Guidance: Enhancing Communication and Guidance Development Practices
The US Food and Drug Administration (USFDA) has released two insightful reports aimed at improving its internal practices and...
Sharan Murugan
Dec 4, 20242 min read
22 views
0 comments


USFDA MD Guidance: Enhancing Safety and Efficiency in Orthopedic Devices and Sterilization Processes
The USFDA Center for Devices and Radiological Health has recently issued three key final guidance documents focusing on orthopedic...
Sharan Murugan
Nov 27, 20242 min read
11 views
0 comments
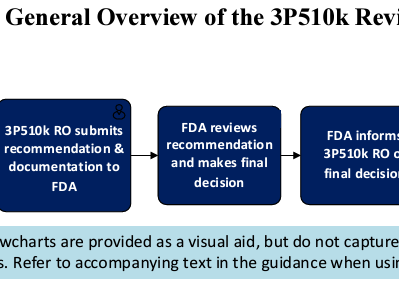
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...
Sharan Murugan
Nov 20, 20242 min read
22 views
0 comments


USFDA Guidance: Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutics
This USFDA draft guidance" Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutic s" , issued on 15 November 2024, offers...
Sharan Murugan
Nov 16, 20242 min read
39 views
0 comments

USFDA Guidance: Study Data Technical Conformance Guide - Technical Specifications
The Study Data Technical Conformance Guide (SDTCG) from the USFDA provides a framework to help sponsors submit standardized study data...
Sharan Murugan
Nov 9, 20242 min read
57 views
0 comments


USFDA Guidance: M13A Bioequivalence for Immediate-Release Solid Oral Dosage Forms
Recently 30th October, 2025 the U.S. Food and Drug Administration (FDA) issued guidance titled " M13A: Bioequivalence for...
Sharan Murugan
Nov 1, 20242 min read
28 views
0 comments
